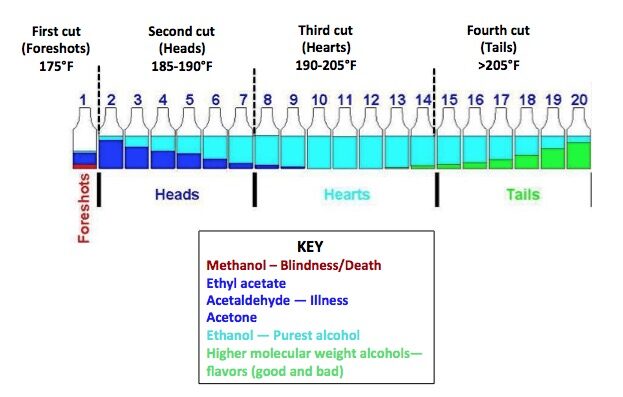
During the grain fermentation process, many compounds are created, some good and some bad, and some of these “bad” compounds are not only harmful but even poisonous. It is the Distillers job to remove the bad compounds and keep the good ones. They call this process simply “Making the Cuts.”
The tool that the Distiller uses to make these cuts is called “distillation.” Technically they use temperature to do all the heavy lifting, as each compound has a different boiling point. The “heads” (the bad stuff) evaporate first followed by the “hearts” (the tasty part) and then finally the “tails” (the mostly bad flavors) are boiled off.
Heads
All the compounds in this group are toxic, one of which is methanol and if drunk can make you blind due to direct toxicity of formic acid on the retina and can even kill you in high concentrations.
Hearts
This section of the distillation process is also called “low wine” contains most of the aromatics–fruits, florals, and some sweet spices, created in fermentation thanks to the yeast that the distiller selects to use.
Tails
These are also referred to as “fusel oils” and are the last compounds to evaporate during distillation. Most of the compounds are undesirable for use in the finished product, however some are at the discretion of the distiller. The selected portion of the tails that the distiller wants to use are collected then condensed back into the low wine then redistilled into the “high wine” using the second still called a “doubler.”
HEADS
68.36F Acetaldehyde –CH3CHO
- Odor of green apples or grass
- Highly flammable
- Used as a solid fuel in portable stoves
- Also used in slug pellets for your garden
132.8F Acetone –CH3COCH3
- Distinct chemical odor
- Highly volatile
- Nail polish remover
- Industrial solvent used in making plastics
148.64F Methanol –CH3OH
- Sweet alcohol odor
- Toxic, causes blindness if drank
- Another industrial solvent, used in making resins
- Used to be distilled from wood, therefore also referred to as “wood alcohol“
170.78F Ethyl Acetate –C2H5OOCCH3
- Extremely distinct scent, will make your eyes water
- Killing agent used by entomologists in jars and traps for collecting insects
- Also used to decaffeinate coffee
HEARTS
173.12F Ethyl Alcohol –C2H5OH
- Familiar fragrance of a tavern or bar
- The desired portion of the distillation process
- Depending on grains in the mashbill used, can be aged to become Bourbon, rye, malt, or other whisk(e)y styles
- Also used (at higher proof) as industrial-grade alcohol in a variety of manufacturing processes, like the manufacture of synthetic rubber, which is why virtually all the distilleries in America were converted to making industrial alcohol during World War II.
TAILS
206.96F Propyl Alcohol –C3H7OH
- Slightly floral and/or medicinal scent
- A solvent in some cosmetics and used in making lacquers
212F Water –H2O
- Should be odorless
- Should not contain iron as that will make the whisk(e)y gray in color
243.5F Butyl Alcohol –C4H9OH
- Lightly sweet scent
- Used as a base for making perfumes, can be used as a paint thinner
244.4F Acetic Acid –CH3COOH
- Smells like vinegar because it is vinegar
- Many uses from salad dressings and as a pickling agent to general purpose cleaning products
280.04F Amyl Alcohol –C5H11OH
- Odor of bananas or Band-Aids
- Variety of uses as an industrial solvent
- Also used to make amyl acetate which can be used as a flavoring agent tasting like banana or apple
321.8F Furfural –C5H4O2
- Almond odor
- Colorless liquid that darkens when exposed to air
- One of the aromatic components of vanilla, flowers and beans
- Important chemical solvent, also found in many processed foods